44 open-label study advantages and disadvantages
External and internal validity of open label or double-blind ... - PubMed In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-randomized treatment decisions and on reporting of outcomes. However, a blinded trial is not always feasible. Understanding Clinical Trial Terminology: What is an Open Label ... Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ...
External and internal validity of open label or double‐blind trials in ... Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias.

Open-label study advantages and disadvantages
External and internal validity of open label or double-blind trials in ... disadvantages of open-label or double-blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-random- PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub- What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded.". If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open.". After recruitment to the trial, the participants were allocated to treatment using block randomisation.
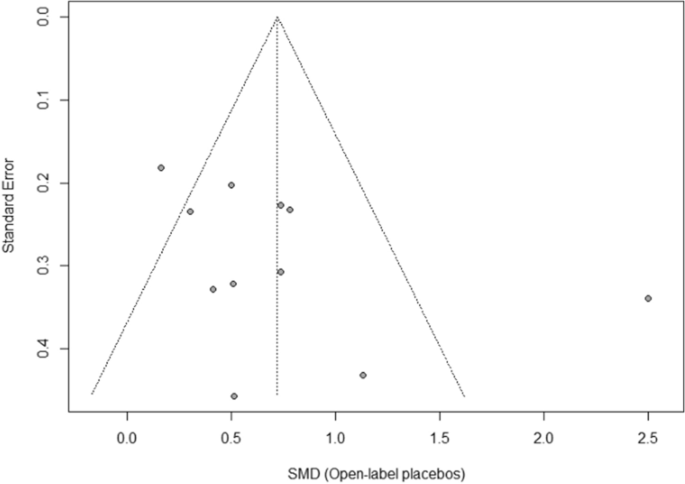
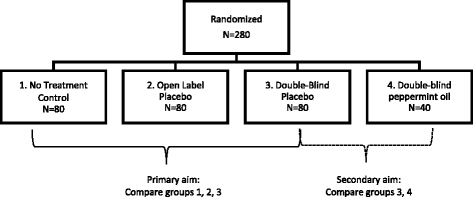
Open-label study advantages and disadvantages. External and internal validity of open label or double ... - ResearchGate a blinded trial may be less subject to bias than an open-label trial because it minimizes the impact of knowledge of treatment allocation on postrandomized treatment decisions and on reporting of... Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: • Open-Label Trial - an overview | ScienceDirect Topics An open-label trial has no blinding: everyone knows which patient is receiving which treatment. Open-label studies lack the rigor of blinded studies. (PDF) What is an open label trial? - ResearchGate The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to...
Open-label extension studies: do they provide meaningful ... - PubMed Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised access to the drug from consumers and their physicians. White Privilege: Advantages and Disadvantages | Free Essay Example White Privilege: Advantages and Disadvantages. Topic: Sociology Words: 848 Pages: 3 Sep 8th, 2022. White privilege is the unmerited benefit that white people experience because they are not exposed to racism. These advantages are frequently undetectable to white people since they perceive freedom as a guaranteed action, which everybody encounters. 16 Advantages and Disadvantages of a Double-Blind Study The FDA even notes that the placebo response is steadily growing in the general population. This disadvantage creates another limitation where the structure of a double-blind study may not provide useful information. 9. Double-blind studies are an expensive effort to pursue. Study designs — Centre for Evidence-Based ... - University of Oxford An experimental comparison study in which participants are allocated to treatment/intervention or control/placebo groups using a random mechanism (see randomisation). Best for study the effect of an intervention. Advantages: unbiased distribution of confounders; blinding more likely; randomisation facilitates statistical analysis.
Crossover trials: what are they and what are their advantages and ... One can say that study participants serve as their own control. This leads to another advantage which is less study participants are required compared to a standard parallel randomized controlled trial (RCT). Reduction of sample size is consistent with the principle in medical research to use resources wisely. Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. 14 Advantages and Disadvantages of a Randomized Controlled Trial - Vittana List of the Disadvantages of Randomized Controlled Trials 1. The logistics of a randomized controlled trial can be demanding. Researchers and participants may need to endure a long trial run to ensure that there is enough data for comparison.
Advantages and Disadvantages of the Modes of Transportation Evaluation: Road. One of the major advantages of the road transport is its cost-effectiveness in short-distance delivery. It is fast and can be used for door-to-door delivery. The infrastructure is well-developed, and the construction of roads requires comparatively low investment and short periods.
What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded.". If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open.". After recruitment to the trial, the participants were allocated to treatment using block randomisation.
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub-
External and internal validity of open label or double-blind trials in ... disadvantages of open-label or double-blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-random-















![PDF] Long-acting, injectable antiretroviral therapy for the ...](https://d3i71xaburhd42.cloudfront.net/274c51f88b6396ab37b2f6c6c3b31b9d302c7643/2-Table2-1.png)
![PDF] The Clinical Viewpoint: Definitions, Limitations of ...](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)










Post a Comment for "44 open-label study advantages and disadvantages"