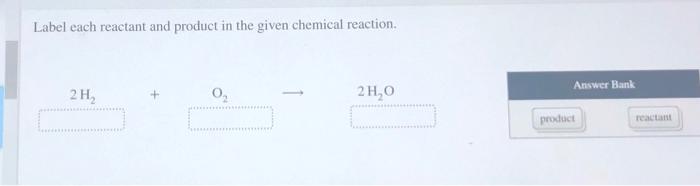
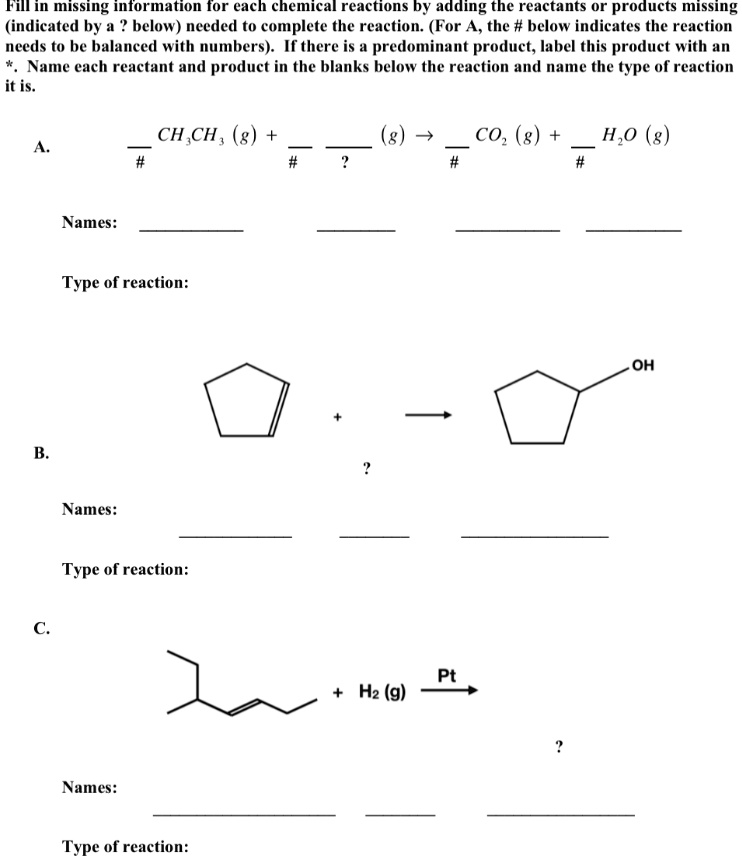
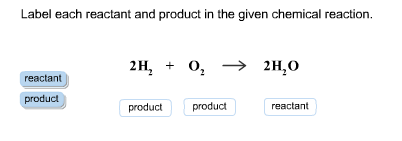
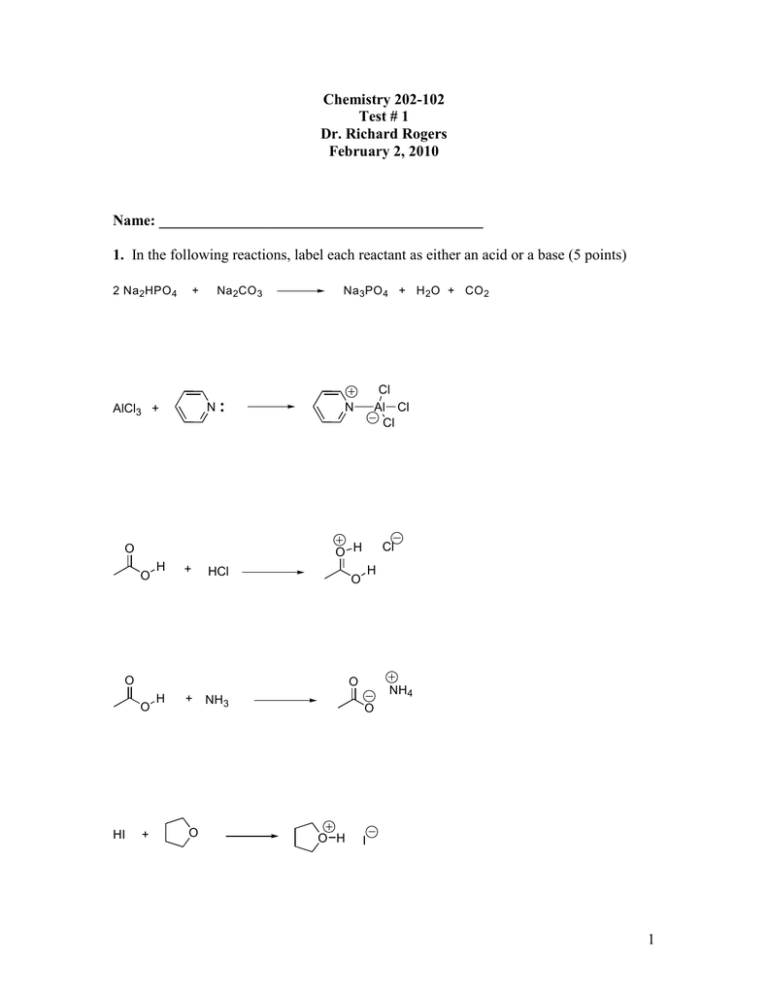
45 label each reactant and product in the given chemical reaction
en.wikipedia.org › wiki › Half-lifeHalf-life - Wikipedia For a first-order reaction, the half-life of a reactant is independent of its initial concentration. Therefore, if the concentration of A at some arbitrary stage of the reaction is [A], then it will have fallen to 1 / 2 [A] after a further interval of (ln 2)/k. Hence, the half-life of a first order reaction is given as the following: 6.7: Balancing Chemical Equations - Chemistry LibreTexts Determine the correct chemical formulas for each reactant and product. Write the skeleton equation. Count the number of atoms of each element that appears as a reactant and as a product. If a polyatomic ion is unchanged on both sides of the equation, count it as a unit.
› questions-and-answers › stateAnswered: State Suppose that a gas obeys the van… | bartleby The reactant, a conjugated enone undergoes an addition reaction by the… question_answer Q: The free energy change for the following reaction at 25 °C, when [H+] = 1.15 M and [Co²+] = 0.00897…
Label each reactant and product in the given chemical reaction
chem.libretexts.org › Bookshelves › General_Chemistry15.2: The Equilibrium Constant (K) - Chemistry LibreTexts Aug 14, 2020 · Given: balanced equilibrium equation, \(K\) at a given temperature, and equations of related reactions. Asked for: values of \(K\) for related reactions. Strategy: Write the equilibrium constant expression for the given reaction and for each related reaction. From these expressions, calculate \(K\) for each reaction. Solution: 4.E: Chemical Reactions and Equations (Exercises) From the statement "sodium metal reacts with water to produce sodium hydroxide and hydrogen," identify the reactants and the products. From the statement "magnesium hydroxide reacts with nitric acid to produce magnesium nitrate and water," identify the reactants and the products. Answered: Label each reactant as either a… | bartleby Science Chemistry Q&A Library Label each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base. a. HF (aq) + H2O (l) --> H3O+ (aq) + F- (aq) HF (aq) ["Bronsted-Lowry acid", "Bronsted-Lowry base"] H2O (l) ["Bronsted-Lowry acid", "Bronsted-Lowry base"] b.
Label each reactant and product in the given chemical reaction. › questions-and-answers › 4Answered: 4. Formulate the potential product of… | bartleby Transcribed Image Text: 4. Formulate the potential product of each of the following reactions. Write "no reaction" where appropriate. Determine the R/S designation for both starting materials and products in the following SN2 reactions. Solved Label each reactant and product in the given chemical | Chegg.com Question: Label each reactant and product in the given chemical reaction. Nt 3H, 2 NH Automobile airbags inflate due to the formation of nitrogen gas from the chemical reaction 2 NaN, (s) + 3N, (B) + 2 Na (s) Identify the number of each atom in the reactants and products for this balanced reaction. › read › science8.3 Le Chatelier's principle | Chemical equilibrium | Siyavula The forward reaction is favoured by higher pressures because there are \(\text{2}\) gas molecules of product for every \(\text{4}\) gas molecules of reactant. Refer to Chapter 14 for more information on the Haber process and other industrial applications. CH104: Chapter 5 - Chemical Reactions - Chemistry 5.4 Some Types of Chemical Reactions. Although there are untold millions of possible chemical reactions, most can be classified into a small number of general reaction types. Classifying reactions has two purposes: it helps us to recognize similarities among them, and it enables us to predict the products of certain reactions.
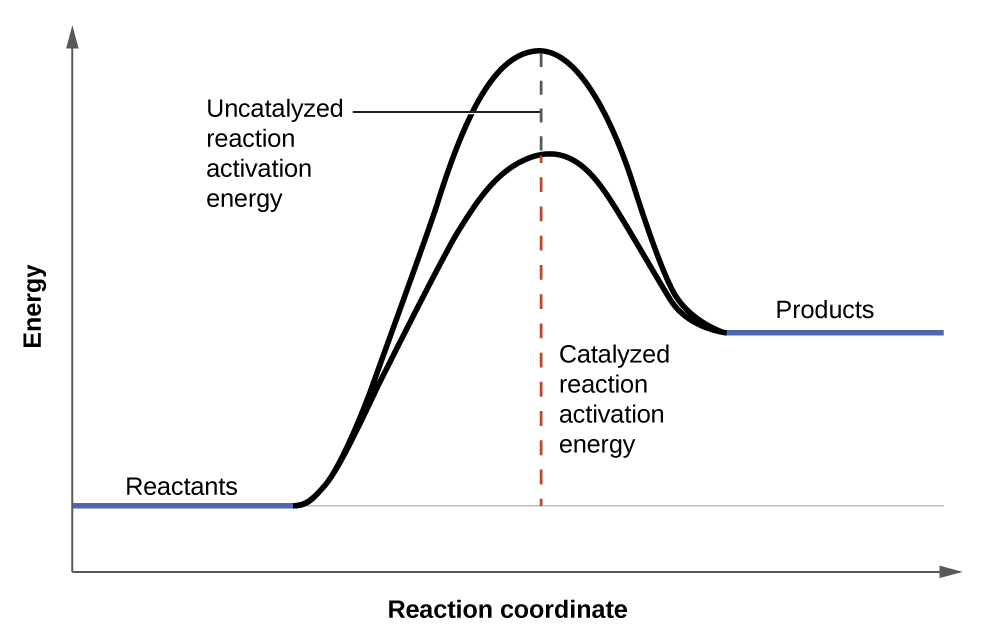
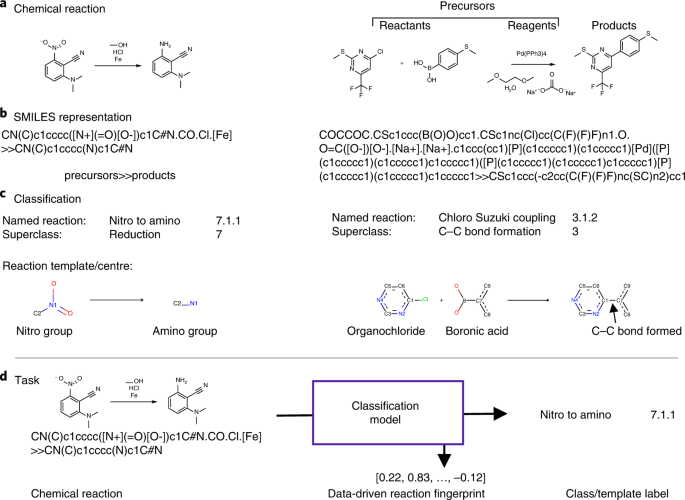
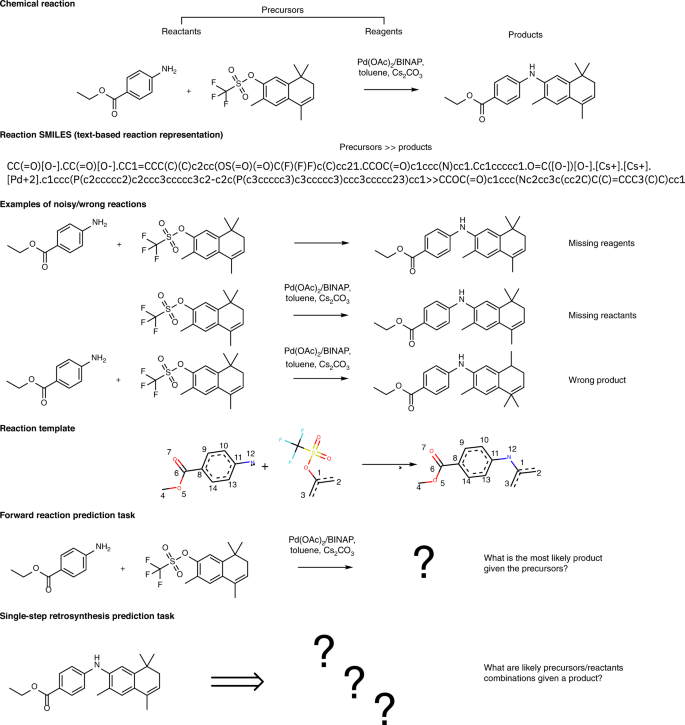
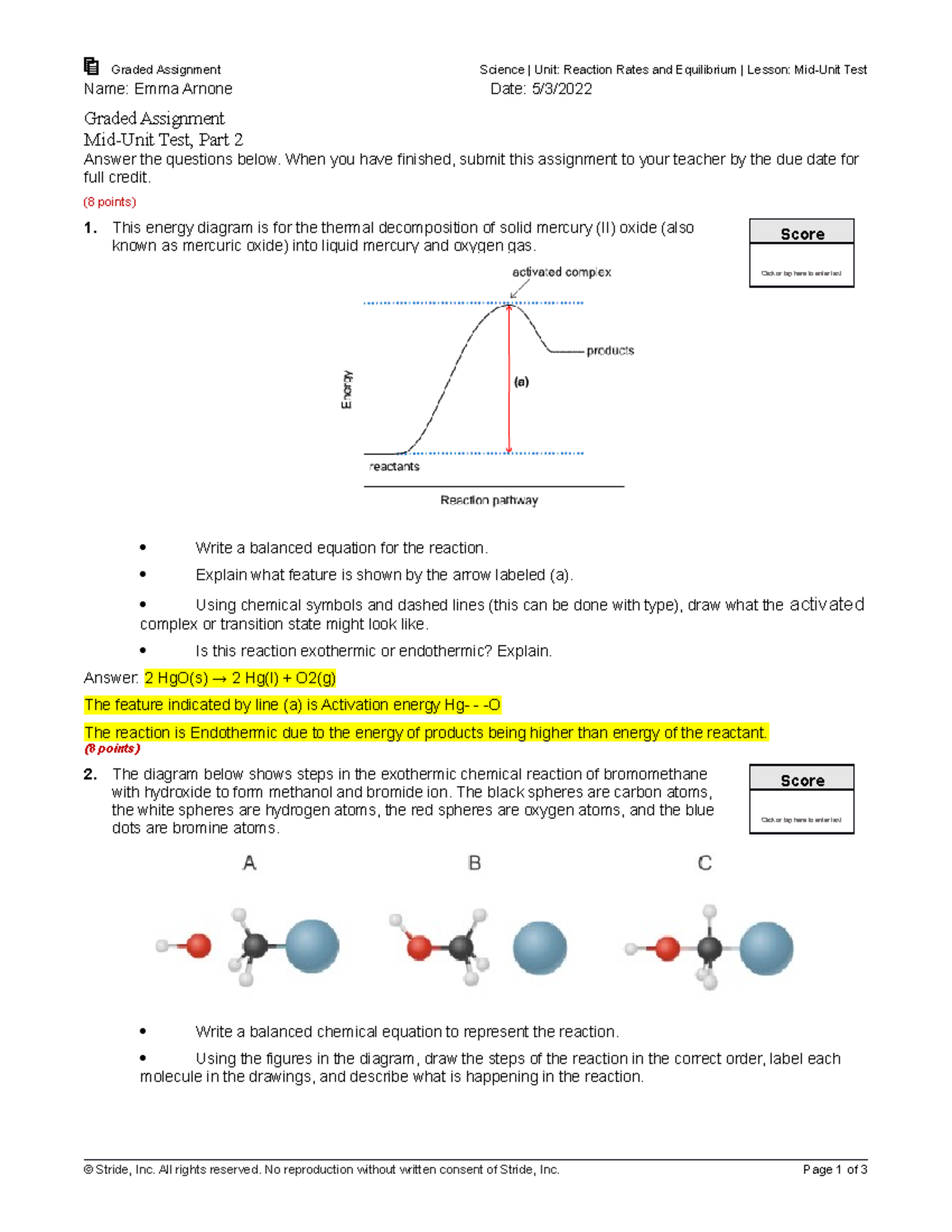
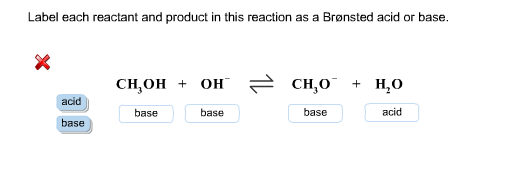
chem.libretexts.org › Bookshelves › Physical_and2.5: Reaction Rate - Chemistry LibreTexts Jun 17, 2022 · The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the products per unit time. The speed of a chemical reaction may be defined as the change in concentration of a substance divided by the time interval during which this change is observed: daylight.com › dayhtml › docDaylight Theory: SMILES Component parts of a reaction are handled by introducing the ">" character as a new separator. Any reaction must have exactly two > characters in it. ">>" is a valid reaction SMILES for an empty reaction. Each of the ">"-separated components of a reaction must be a valid molecule SMILES. As an aside, molecule SMILES never have a ">" character. Answered: 1 Label each reactant and product in… | bartleby Q: Label each reactant and product in this reaction as a Brønsted acid or base. CH3OH+OH−↽−−⇀CH3O−+H2O. A: Interpretation: The given each reactant and product of the reaction are to be labelled as a Bronsted… Lab Report Types of Reactions.pdf - Neel Raje Ms. Missig... Reaction 4: Heat Sodium Carbonate Ø Use a small spatula to place about half of an inch of sodium carbonate in a clean, dry test tube. Ø Record the appearance (colors, texture) of the reactant in the data table. Ø Label each reactant type. Then, choose the type of reaction you expect for the reactants.
Worked example: Calculating amounts of reactants and products All right, now first let's just set up the reaction. So, this is going to be, we have glucose, so that is C6H12O6, is going to react with oxygen. Now, oxygen in its molecular form is going to be O2. And what it gives is carbon dioxide and water. Plus water. Now the next question is are we balanced. Do we have a conservation of mass here? Reactants and Products in Chemical Reactions - dummies They indicate the number of each chemical species that reacts or is formed. Methane and oxygen (oxygen is a diatomic — two-atom — element) are the reactants, while carbon dioxide and water are the products. All the reactants and products are gases (indicated by the g's in parentheses). In this reaction, all reactants and products are invisible. 4.4: Reaction Yields - Chemistry LibreTexts Identifying the limiting reactant involves comparing the amount of product expected for the complete reaction of each reactant. Each reactant amount is used to separately calculate the amount of product that would be formed per the reaction's stoichiometry. The reactant yielding the lesser amount of product is the limiting reactant. Answered: Classify each reactant and product in… | bartleby Homework help starts here! ASK AN EXPERT. Science Chemistry Q&A Library Classify each reactant and product in this reaction as an acid or base according to the Brønsted theory. HF+H2O↽−−⇀F−+H3O+. Classify each reactant and product in this reaction as an acid or base according to the Brønsted theory. HF+H2O↽−−⇀F−+H3O+.
How would you label each formula in the chemical equation below as ... How would you label each formula in the chemical equation below as either a reactant or a product? F e + S → F eS Chemistry Chemical Reactions Chemical Reactions and Equations 1 Answer anor277 Jan 23, 2017 We designate the reactants as written........ Explanation: The reactants are on the LEFT HAND SIDE of the equation.
Organic Chemistry Q&A.docx - 1. Label each reactant... 1. Label each reactant according to its role (or roles) in the chemical reaction. Check all that apply. 2. Add curved arrows to the reactant side of the SN2SN2 reaction shown. 3. Select the properties of the SN2SN2 reaction mechanism. 4. Draw the major organic product of the reaction.
) In the reaction below, label each reactant as a nucleophile or an ... Answered step-by-step ) In the reaction below, label each reactant as a nucleophile or an electrophile. CH3COO- + O2S (OCH3)2 → CH3COOCH3 + CH3SO4- Chemistry Science Organic chemistry Answer & Explanation Solved by verified expert All tutors are evaluated by Course Hero as an expert in their subject area. CH3COO- ................> nucleophile
The Chemical Equation | Introductory Chemistry - Lumen Learning Example 1. Write and balance the chemical equation for each given chemical reaction. Hydrogen and chlorine react to make HCl. Ethane, C 2 H 6, reacts with oxygen to make carbon dioxide and water.; Solution. Let us start by simply writing a chemical equation in terms of the formulas of the substances, remembering that both elemental hydrogen and chlorine are diatomic:
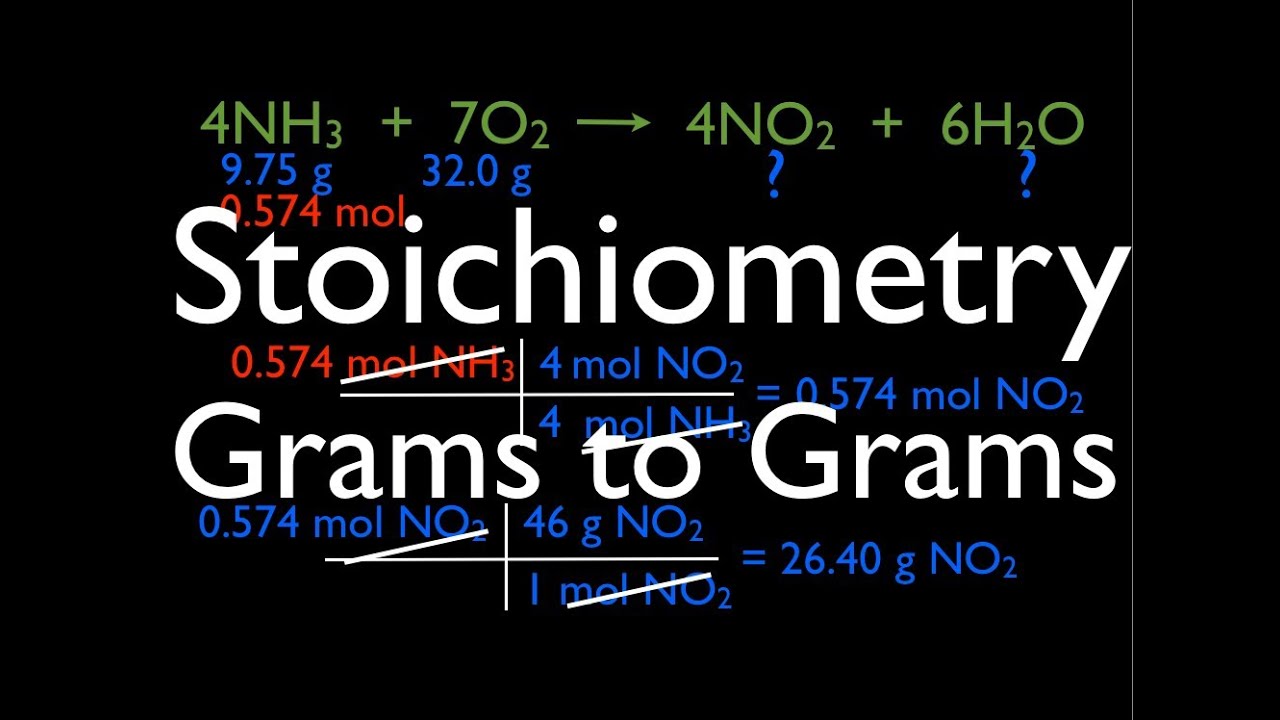
Calculating Limiting Reactant of a Chemical Reaction - ThoughtCo grams H 2 O = (96 x 1/32 x 2 x 18) grams H 2 O. grams H 2 O = 108 grams O 2 O. Much more water is formed from 20 grams of H 2 than 96 grams of O 2. Oxygen is the limiting reactant. After 108 grams of H 2 O forms, the reaction stops. To determine the amount of excess H 2 remaining, calculate how much H 2 is needed to produce 108 grams of H 2 O.
Chemical Equation | Reactants And Products In Chemical Reactions - BYJUS We will first take the maximum number of atoms present on either side of the reaction, that we can find on product side. (Oxygen = 4). Then we multiply the number of oxygen atoms on reactant side by 4, such that the number of oxygen atoms on both sides of the reaction is balanced. The equation becomes F e + 4 H 2 O → F e 3 O 4 + H 2
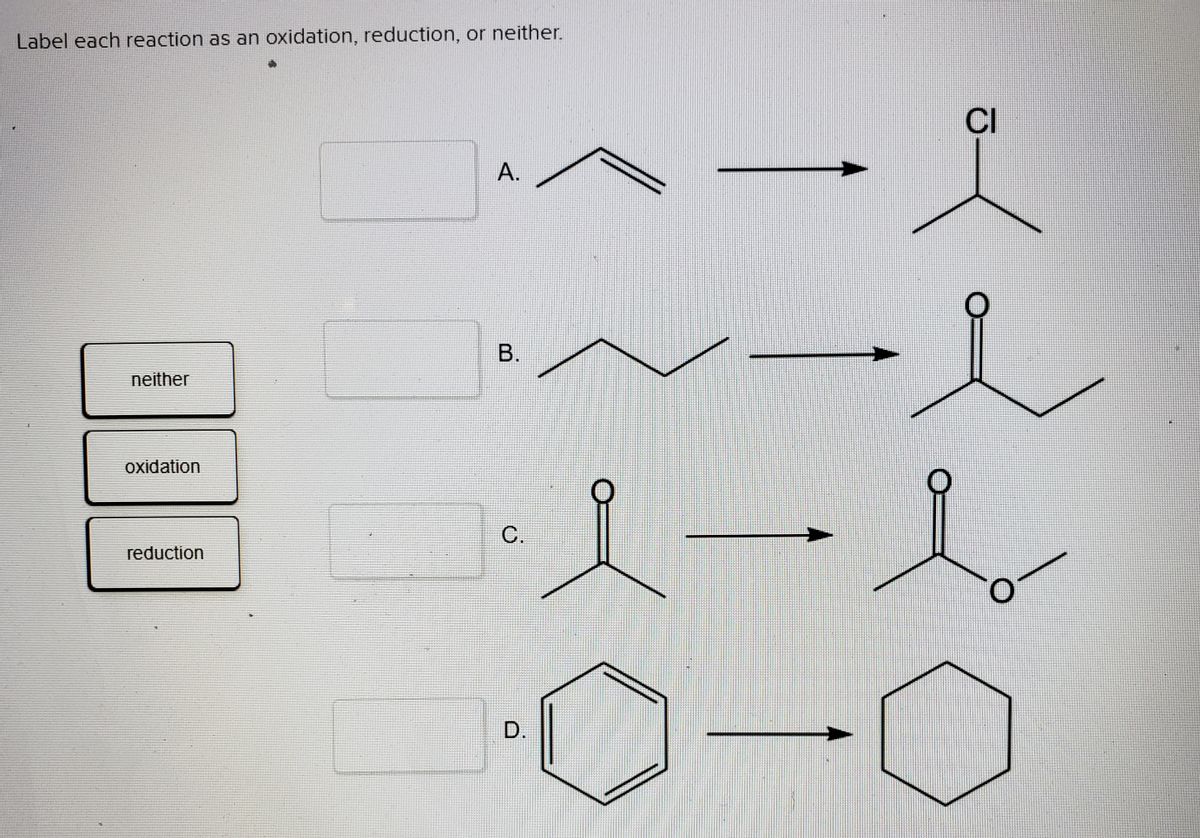
Answered: Label each reaction as an oxidation,… | bartleby Q: Draw the product of reaction B. Select Draw Rings Groups More Erase Reaction B. 1. HNO3, H2SO4 2.… A: The products of the given reactions are to be predicted. Benzene on reaction with nitric acid in…
8.2 How do we represent chemical reactions? - Siyavula 1. Word equations. In mathematic equations we use an equal sign (=) for example 2 + 2 = 4, but in scientific chemical equations, we use an arrow (→), for example C + O 2 → CO 2. When we represent a chemical reaction in terms of words, we write a word equation. For example, when hydrogen gas reacts with oxygen gas to form water, we can write ...
8.5: Limiting Reactant and Theoretical Yield - Chemistry LibreTexts Each reactant amount is used to separately calculate the amount of product that would be formed per the reaction's stoichiometry. The reactant yielding the lesser amount of product is the limiting reactant. For the example, in the previous paragraph, complete reaction of the hydrogen would yield: molHClproduced = 3molH2 × 2molHCl 1molH2 = 6molHCl
14.2: Rate of a Chemical Reaction - Chemistry LibreTexts The reaction rate can also be calculated from the concentrations of aspirin at the beginning and the end of the same interval, remembering to insert a negative sign, because its concentration decreases: rate ( t = 0 − 2.0 h) = − [aspirin]2 − [aspirin]0 2.0h − 0h = − (5.51 × 10 − 3 M) − (5.55 × 10 − 3 M) 2.0 h = 2 × 10 − 5 M/h
Solved 1. Label each reactant and product in the given | Chegg.com See the answer 1. Label each reactant and product in the given chemical reaction. CH4 + 2O2 CO2+ 2H2OCH4 + 2O2 CO2 + 2H2O Answer Bank: Product/Reactant 2. Use the law of conservation of mass to answer the questions. Consider a hypothetical reaction in which A and B are reactants and C and D are products.
Solved Label each reactant and product in the given chemical | Chegg.com Expert Answer 89% (28 ratings) CH4=REACTANT O2 = REACT … View the full answer Transcribed image text: Label each reactant and product in the given chemical reaction. CH_4 + 2O_2 rightarrow CO_2 + 2H_2O Previous question Next question
SOLVED:Label each reactant as a Brønsted-Lowry acid or a ... - Numerade Now with bronze did Lowry acid based definitions. Then we end up creating a conjugate acid and conjugate base. On the product side, the acid produces a conjugal base and the base produces a conjugate acid. In the next chemical reaction, we see that C H three n h two is accepting the hydrogen ion.
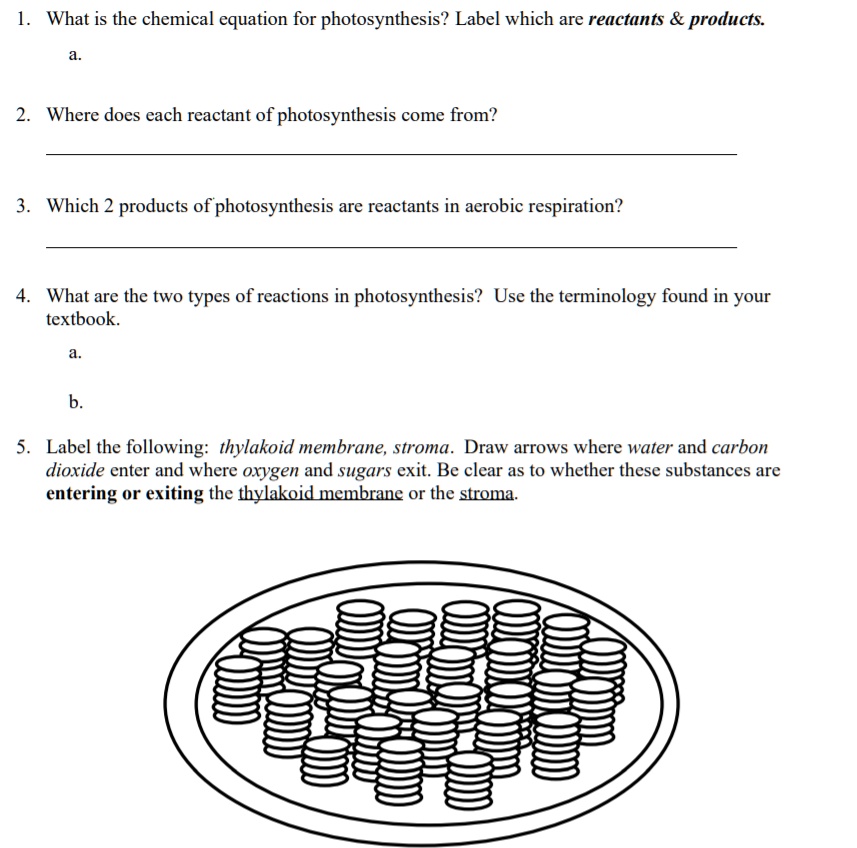
Reactants and Products - GeeksforGeeks The reactants in the formation of water are hydrogen (H2) and oxygen (O 2) gas. Water is the product (H 2 O). Carbon dioxide (CO 2) and water are the reactants in photosynthesis (H 2 O). Glucose is the product (C 6 H 12 O 6 ). It should be noted that sunlight is not a reactant. Reactants are matter (atoms, molecules, and ions) rather than energy.
Answered: Label the following parts of the… | bartleby Label the following parts of the chemical equation below: coefficient, subscript, products, reactants, yields, chemical formula. A. B. A. С. В. C. CaCl, + H,0 Cao + 2HCI D. E. D. E. F. F. The Law of Conservation of Mass states that during a chemical reaction, mass/matter is not Because of this law, there must be exactly Jon each side of a ...
7.2 Reactants and products | Chemical reactions | Siyavula REACTANTS (before the reaction) → PRODUCTS (after the reaction) Do you see how the atoms have rearranged? This means a chemical reaction has taken place. Label the diagram with 'reactants' and 'product'. The reaction between carbon and oxygen takes place when we burn coal. Coal is carbon and when it burns in oxygen gas, carbon dioxide is formed.
Answered: Label each reactant as either a… | bartleby Science Chemistry Q&A Library Label each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base. a. HF (aq) + H2O (l) --> H3O+ (aq) + F- (aq) HF (aq) ["Bronsted-Lowry acid", "Bronsted-Lowry base"] H2O (l) ["Bronsted-Lowry acid", "Bronsted-Lowry base"] b.

for each reaction listed identify the proton donor or acid and the proton acceptor or base label eac
4.E: Chemical Reactions and Equations (Exercises) From the statement "sodium metal reacts with water to produce sodium hydroxide and hydrogen," identify the reactants and the products. From the statement "magnesium hydroxide reacts with nitric acid to produce magnesium nitrate and water," identify the reactants and the products.
chem.libretexts.org › Bookshelves › General_Chemistry15.2: The Equilibrium Constant (K) - Chemistry LibreTexts Aug 14, 2020 · Given: balanced equilibrium equation, \(K\) at a given temperature, and equations of related reactions. Asked for: values of \(K\) for related reactions. Strategy: Write the equilibrium constant expression for the given reaction and for each related reaction. From these expressions, calculate \(K\) for each reaction. Solution:






















![Add URI prefix legend below view [#2472479] | Drupal.org](https://www.drupal.org/files/issues/Screen%20Shot%202015-07-02%20at%2018.05.35.png)
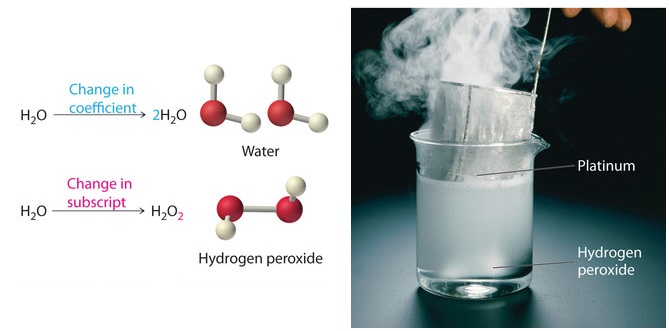

Post a Comment for "45 label each reactant and product in the given chemical reaction"